
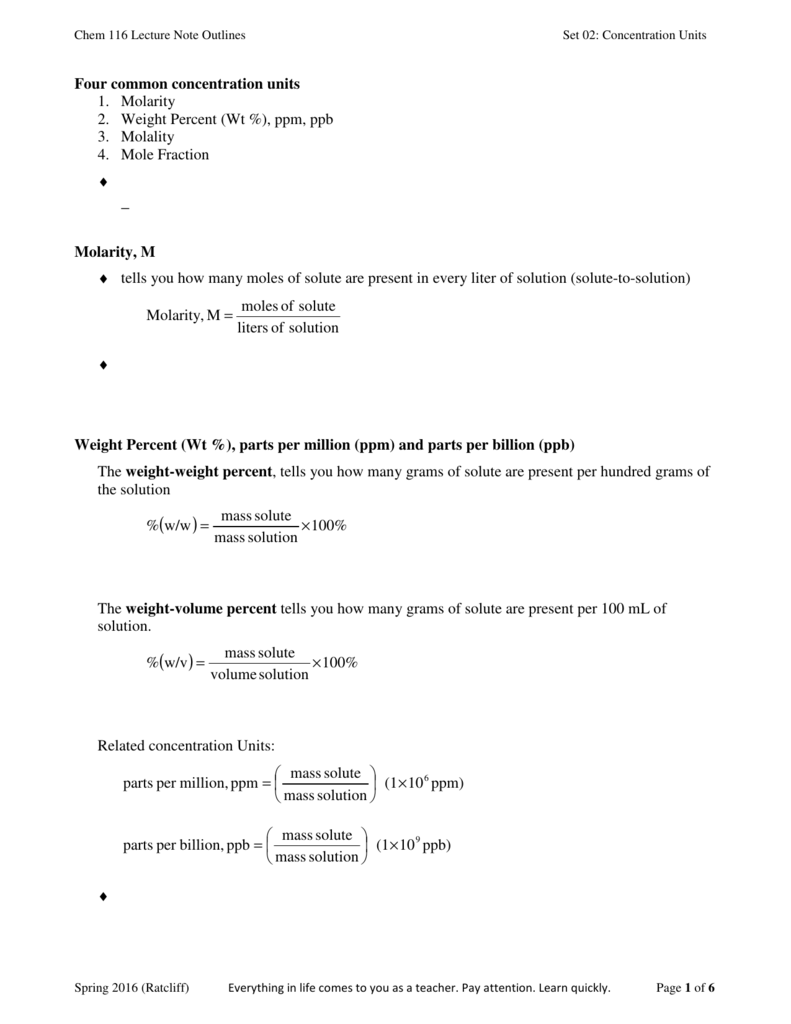
Jan 2008 - 38 What is the concentration of O 2(g), in parts per million, in a solution that contains 0.008 gram of O 2(g) dissolved in 1000. grams of a solution having a concentration of 5 parts per million? June 2009- 36 What is the total mass of solute in 1000. June 2008- 38 What is the concentration of O 2(g), in parts per million, in a solution that contains 0.008 gram of O 2(g) dissolved in 1000. June 2005 -42 What is the concentration of a solution, in parts per million, if 0.02 gram of Na 3PO 4 is dissolved in 1000 grams of water? grams of H 2O, what is the concentration of the resulting solution, in parts per million? If 0.025 gram of Pb(NO 3) 2 is dissolved in 100. Here was another one, but it was a multiple choice. 1 mg/kg 1 part per million ppm can be also be expressed as. It is technically wrong not to add the solute and solvents together to determine the mass of solution. s molar mass, volume or mass of solution (mole, m 3, ft 3, kg, lb m) In the metric system ppm for mass can be expressed in terms of milligram versus kg where. Even though mathematically it didn't change a thing. Īnswer-The equation above reads "grams of solution", you had to add the solute to the solvent in the denominator. Your response must include both a correct numerical setup and the calculated result. In the space in your answer booklet, calculate the dissolved oxygen concentration of this solution in parts per million. An aqueous solution has 0.0070 gram of oxygen dissolved in 1000. Most students lost 1 of the 2 points on this one.Ħ6. Then June 2007, New York State threw this curveball. What is the concentration of a solution, in parts per million, if 0.02 gram of NaCl is dissolved in 1000. This is really just a plug and chug problem. Think about this 1ppm = 1 inch in 16 miles This is used for very small concentrations of particles in solutions. A flask contains 2.00 moles of nitrogen and 2.00 moles of helium.This is another way of determining concentration. 78.00 is to 0.167 as the total pressure is to one, so 468 mmHg is the answer.ħ2. Note that moles cancel out when calculating mole fraction, so it is a unitless value.

The sum of all mole fractions adds up to 1. Mole fraction or molar fraction is the number of moles of one component of a solution divided by the total number of moles of all chemical species.
#How to calculate ppm from mole fraction how to
When the partial pressure of the oxygen is 78.00 mm of mercury, what is the total pressure in the flask?ħ1. How to Calculate Mole Fraction of a Solution. A mixture of 14.0 grams of hydrogen, 84.0 grams of nitrogen, and 2.0 moles of oxygen are placed in a flask. A tank contains 5.00 moles of O 2, 3.00 moles of neon, 6.00 moles of H 2S, and 4.00 moles of argon at a total pressure of 1620.0 mm Hg. Keep in mind that once one partial pressure is calculated, the other can be arrived at by subtraction, if so desired.ħ0. a) How many moles of O 2 are in the tank? A tank contains 480.0 grams of oxygen and 80.00 grams of helium at a total pressure of 7.00 atmospheres. What is the partial pressure of the oxygen?Ħ9.
The atmospheric pressure in the room is 96.00 kPa. 80.0 liters of oxygen is collected over water at 50.0 ☌. If 60.0 L of nitrogen is collected over water at 40.0 ☌ when the atmospheric pressure is 760.0 mm Hg, what is the partial pressure of the nitrogen?Ħ8. Calculate the partial pressure of helium and argon if the total pressure inside the container is 4.00 atm.Ħ6. A container with two gases, helium and argon, is 30.0% by volume helium. What is the total pressure inside the container?Ħ6. The partial pressures of the three gases are 2.00 atm, 3.00 atm, and 4.00 atm, respectively. A container holds three gases: oxygen, carbon dioxide, and helium.


 0 kommentar(er)
0 kommentar(er)
